As a well-recognized expert at phage display technology, Creative Biolabs offers our worldwide clients the best phage display library screening services through our excellent biopanning strategies and state-of-the-art equipment. Our scientists are pleased to tailor the most appropriate strategies to screen the interested libraries of our customers.
According to different demands, a series of targets can be qualified screened by our service. We accept targets provided by clients, but also provide related services to synthesize and express the interested targets.
Phage display technology is one of the major approaches employed in various protein interaction studies, especially in the discovery of specific antibodies, scaffolds or ligands. While binders with the desired specificity generally exist at low frequencies in the constructed libraries. In this way, phage display library screening has become an effective technique for the enrichment and identification of binders with high affinity and specificity from interested libraries.
At Creative Biolabs, the proprietary protocol and tailored biopanning strategy allow the increase of candidates with higher affinity and desired specificity (e.g. binders recognize conformational epitope rather than linear epitope). In general, by conducting four rounds library screening, our scientists can select scFv/Fab antibodies with the affinity of 10-7. Through constructing serial sub-libraries of the isolated scFv/Fab antibodies, we can increase the affinity from 10-8 to 10-9. Moreover, Creative Biolabs has successfully obtained a scFv antibody with an extremely high affinity of 10-12.
Based on our standard biopanning protocol, Creative Biolabs can utilize different strategies for library screening to meet our clients’ project goals.
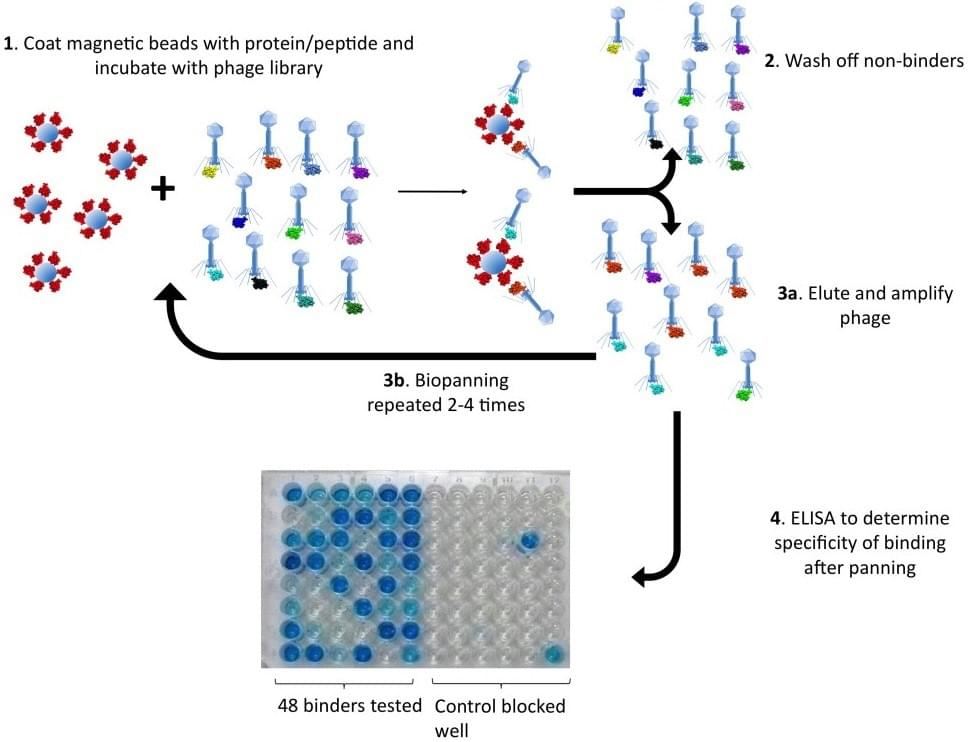
Solid-Phase Screening : the most straightforward and widely used way to select binders against interested target adsorbed onto a solid surface. Typically, the targets can be coated on the well of immunotubes or microtiter plates for isolating specific binders.
Solution-Sorting Screening: an ideal approach to isolate binders recognize naïve targets. The target-binder interaction is carried out in solution with subsequent capture by the appropriate method.
Cell-Based Screening: a method in particular for cell surface antigen selection. It is a reliable approach to screen cell surface antigens with native conformation, especially when the antigen is hardly expressed as recombinant protein.
In vivo Screening: a high throughput method for efficiently isolating tissue-specific binders. The selection is based on binding to target in vivo after the injection of interested phage libraries into whole animals, and then rescuing phage bound to specific organs and tissues.
Ex vivo Screening: a novel technique to employ primary cell suspensions of organs and tissues as targets. All cell types can be exposed for selection to identify the most specific binders.
More featured phage display library screening strategies.
Other Applications of Our Library Screening Service
In terms of years of experience in phage display realm, Creative Biolabs utilizes its advanced technology platforms and specialized expertise to serve our global clients with high-quality library screening services. Our scientists are confident in tailoring our customers the most reliable and cost-effective protocol to facilitate their meaningful research.
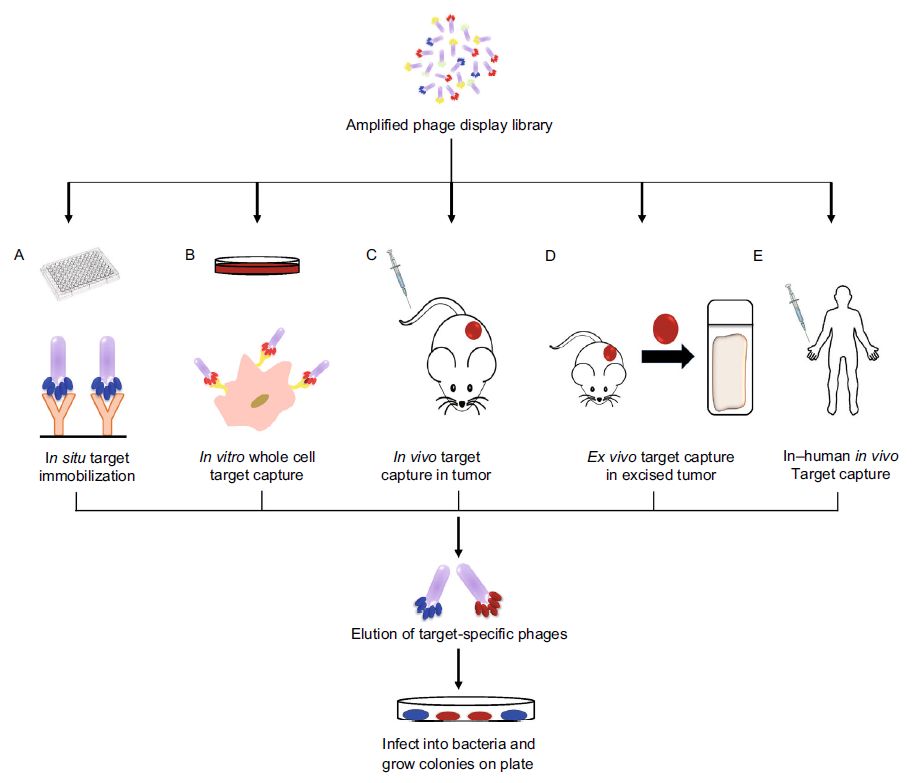 Fig. 1 Various approaches in capturing high affinity peptide through phage display screening. (Phei Er Saw, 2019)
Fig. 1 Various approaches in capturing high affinity peptide through phage display screening. (Phei Er Saw, 2019)
In complex solid tumors, peptides can be used to target dysfunctional tumor vascular systems, dense extracellular matrix, tumor stromal cells, or overexpressed receptors on tumors. Many peptide-based targeting ligands have shown promising results in enhancing solid tumor therapy, including increased tumor accumulation, highly specific tumor targeting, and enhanced tumor inhibition when used in combination with anticancer drugs or biological agents. Phage display technology allows the selection of target-binding peptides with high affinity and selectivity from the complex mixture pool of billions of display peptides on the phage in the combinatorial library and can be further enriched by the biopanning process. This technique can isolate various cancer-specific ligands by in situ, in vitro, in vivo, and ex vivo screening methods.
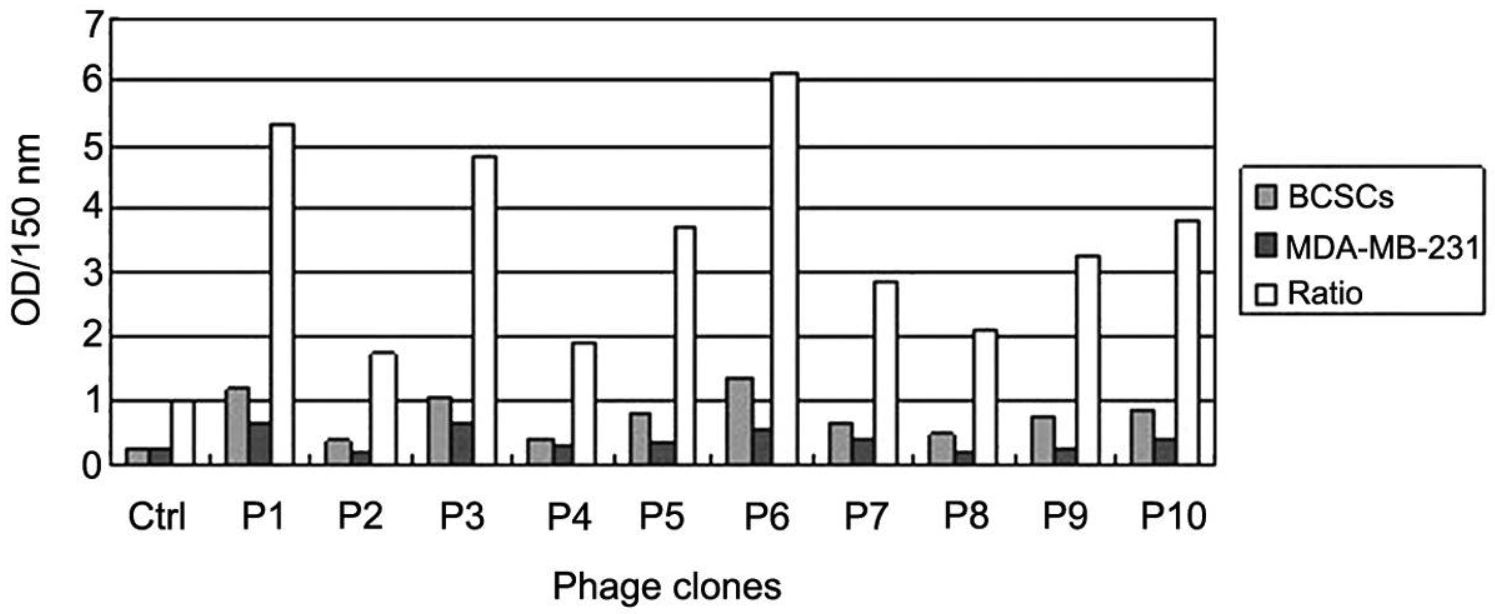 Fig. 2 Identification of phage specificity to breast cancer stem cells by ELISA. OD, optical density; BCSC, breast cancer stem cell. (Fei Liu, 2016)
Fig. 2 Identification of phage specificity to breast cancer stem cells by ELISA. OD, optical density; BCSC, breast cancer stem cell. (Fei Liu, 2016)
Breast cancer stem cells are derived from normal breast stem cells or differentiated breast cancer cells and have the abilities of self-renewal, differentiation, and tumorigenesis. Traditional radiotherapy and chemotherapy can not easily kill breast cancer stem cells, leading to breast cancer recurrence or metastasis. Therefore, based on the phenotypic and biological characteristics of breast cancer stem cells, the study of the technical strategies of breast cancer stem cells may be beneficial to the treatment of breast cancer. The phage display peptide library is an efficient and simple tool that has been widely used in the development of antineoplastic drugs and tumor diagnostic markers. In this study, breast cancer stem cells were isolated from MDA-MB-231 cells by serum-free culture, and then the phage display technique was used to screen the phages that could specifically bind to breast cancer stem cells. It was proven that the positive bacteriophage (named KL-6) screened after three rounds of biological selection showed high affinity and specificity for breast cancer stem cells. After ELISA identification and amplification, the researchers collected 8 positive phages for DNA sequencing and finally obtained the amino acid sequence of the antigen-related ligand peptide on the surface of breast cancer stem cells. These results may provide the basis and mechanism guidance for targeted therapy for breast cancer.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.